Life Sciences Commissioning, Qualification and Validation
Por um escritor misterioso
Last updated 31 julho 2024


Commissioning, Qualification and Validation. - CSV Life Science

Commissioning and Qualification for Life Sciences Facilities
Commissioning, Qualification and Validation (CQV) are requirements of modern facilities within the Life Science industry. Be it a Medical Device

Commissioning, Qualification and Validation: A GMP Approach

COMMISSIONING, QUALIFICATION & VALIDATION UNIVERSITY.

Effective Construction Program and Project Management

What is commissioning, qualification and validation? - National Research Council Canada

COMMISSIONING & QUALIFICATION IN CELL THERAPIES, The Netherlands Affiliate, ISPE

Commissioning & Qualification: Debunking the Myths – No deviation

Commissioning vs Qualification vs Validation in Pharma

Commissioning, Qualification and Validation (CQV) Consultants

Commissioning, Qualification & Validation
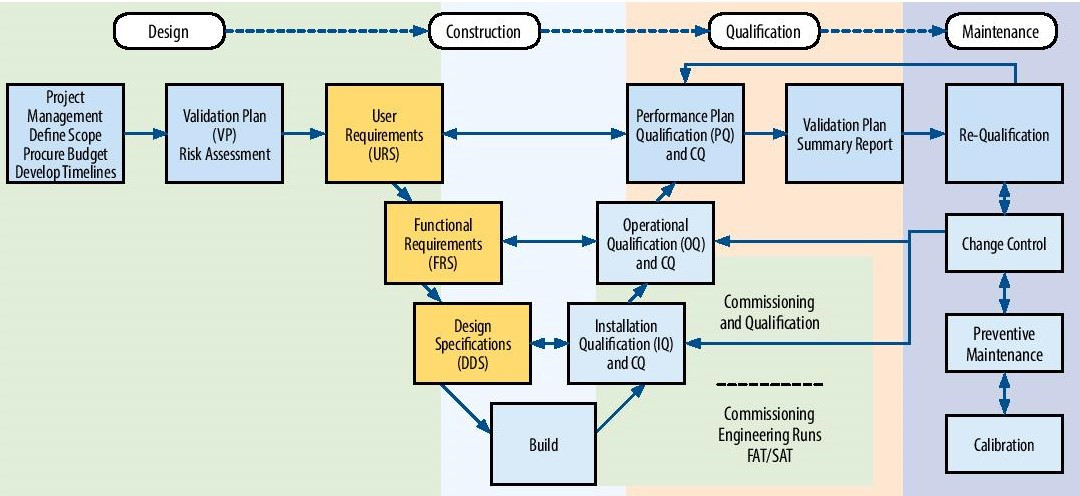
CQV Services - Elomatic India
Biopharma Commissioning & Qualification Services, Consultants
Recomendado para você
-
 Last remaining classic ICQ servers go down. R.I.P. Classic ICQ31 julho 2024
Last remaining classic ICQ servers go down. R.I.P. Classic ICQ31 julho 2024 -
 Amy Wu Adventures (Boxed Set): Amy Wu and the Perfect Bao; Amy Wu and the Patchwork Dragon; Amy Wu and the Warm Welcome; Amy Wu and the Ribbon Dance31 julho 2024
Amy Wu Adventures (Boxed Set): Amy Wu and the Perfect Bao; Amy Wu and the Patchwork Dragon; Amy Wu and the Warm Welcome; Amy Wu and the Ribbon Dance31 julho 2024 -
 Links com abuso infantil ficam no topo de buscas de Google e Bing31 julho 2024
Links com abuso infantil ficam no topo de buscas de Google e Bing31 julho 2024 -
 ICQ Chat-page31 julho 2024
ICQ Chat-page31 julho 2024 -
 icq invite brasil31 julho 2024
icq invite brasil31 julho 2024 -
 ICQ FAQ31 julho 2024
ICQ FAQ31 julho 2024 -
ICQ Expo31 julho 2024
-
 Black and Gold Wedding Invitation Fall Wedding Invites - Hong Kong31 julho 2024
Black and Gold Wedding Invitation Fall Wedding Invites - Hong Kong31 julho 2024 -
 ICQ New: H Arucu Lockscreen, Lockscreen screenshot, Invitations31 julho 2024
ICQ New: H Arucu Lockscreen, Lockscreen screenshot, Invitations31 julho 2024 -
 ICQ Messenger Introduces Group Video Calls, by Dimitry O. Photo31 julho 2024
ICQ Messenger Introduces Group Video Calls, by Dimitry O. Photo31 julho 2024
você pode gostar
-
 Microsoft-Activision deal: EU approves takeover of Call of Duty maker31 julho 2024
Microsoft-Activision deal: EU approves takeover of Call of Duty maker31 julho 2024 -
 Dubladores de sucessos falam sobre a profissão no Santos Festival Geek31 julho 2024
Dubladores de sucessos falam sobre a profissão no Santos Festival Geek31 julho 2024 -
 8 Best Puzzle Games For Nintendo Switch 202031 julho 2024
8 Best Puzzle Games For Nintendo Switch 202031 julho 2024 -
 Review – Twisted Metal (PS1) – Game Complaint Department31 julho 2024
Review – Twisted Metal (PS1) – Game Complaint Department31 julho 2024 -
 TODOS CÓDIGOS DO FRUIT WARRIORS NOVO JOGO DE ONE PIECE ROBLOX NOVOS CÓDIGOS NEW CODES AXIORE31 julho 2024
TODOS CÓDIGOS DO FRUIT WARRIORS NOVO JOGO DE ONE PIECE ROBLOX NOVOS CÓDIGOS NEW CODES AXIORE31 julho 2024 -
LifeAfter - #LifeAter #DeathHighRestart Are you ready to face the challenge again?🏫 How to get a better performance during the rescue event in #DeathHigh, Survivors? Don't forget to share your tips here31 julho 2024
-
 Silver Chariot: Part 1 - Stand Sound Profiles31 julho 2024
Silver Chariot: Part 1 - Stand Sound Profiles31 julho 2024 -
 Miranha por mestre_aprendiz Miranha, Desenhar online, Jogos de desenho31 julho 2024
Miranha por mestre_aprendiz Miranha, Desenhar online, Jogos de desenho31 julho 2024 -
Bolo no tema Minecraft e com recheio - Dona Chica Bolos31 julho 2024
-
Throne and Liberty is a new Korean MMO coming West, and it puts31 julho 2024



