FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 04 setembro 2024

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

Fast-Track Drug Approval, Designed for Emergencies, Is Now Routine - WSJ
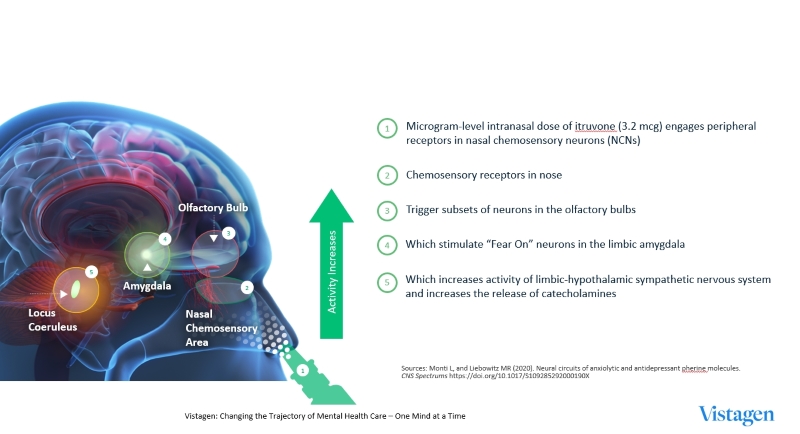
vtgn20230331_10k.htm

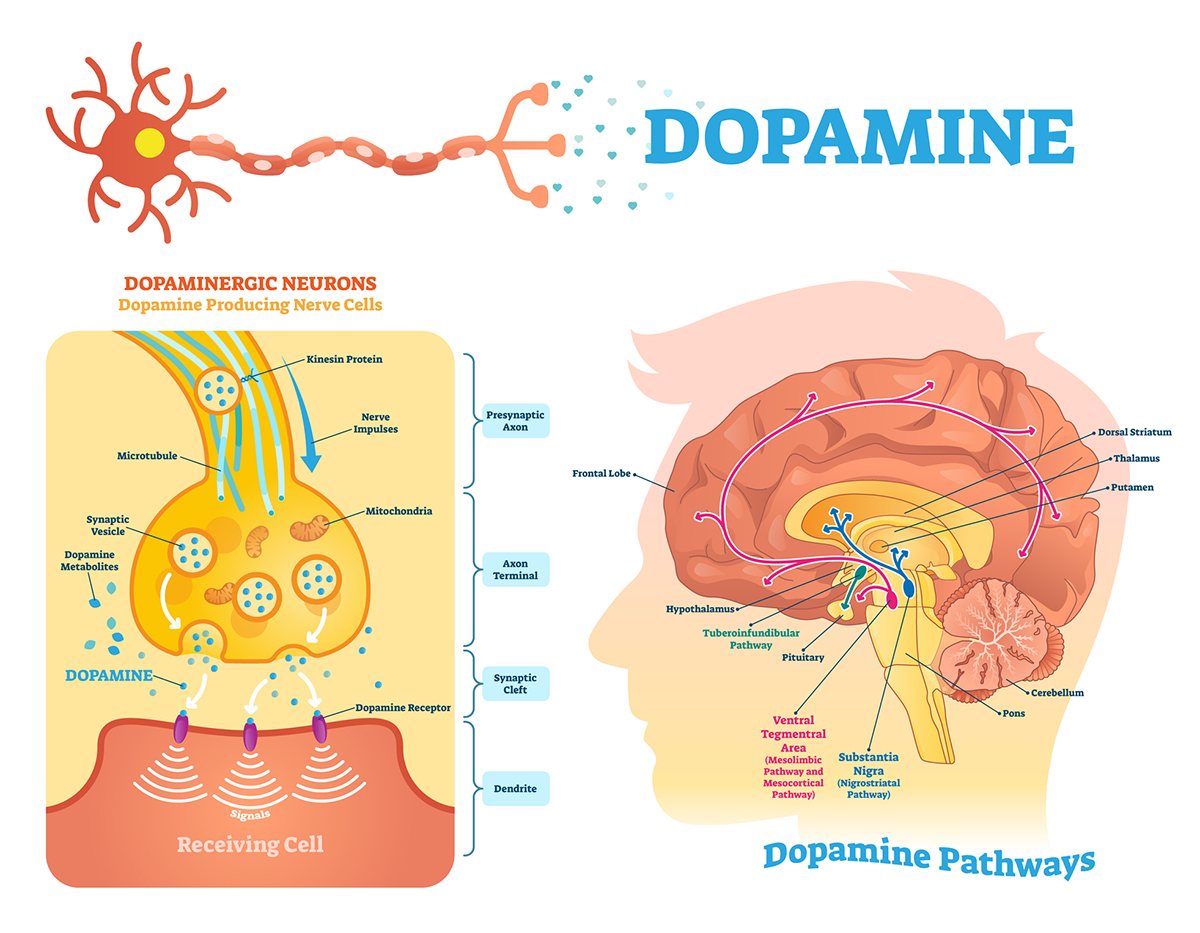
FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services
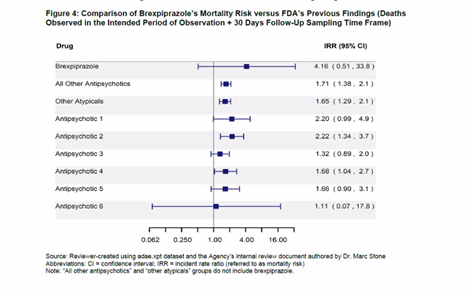
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America
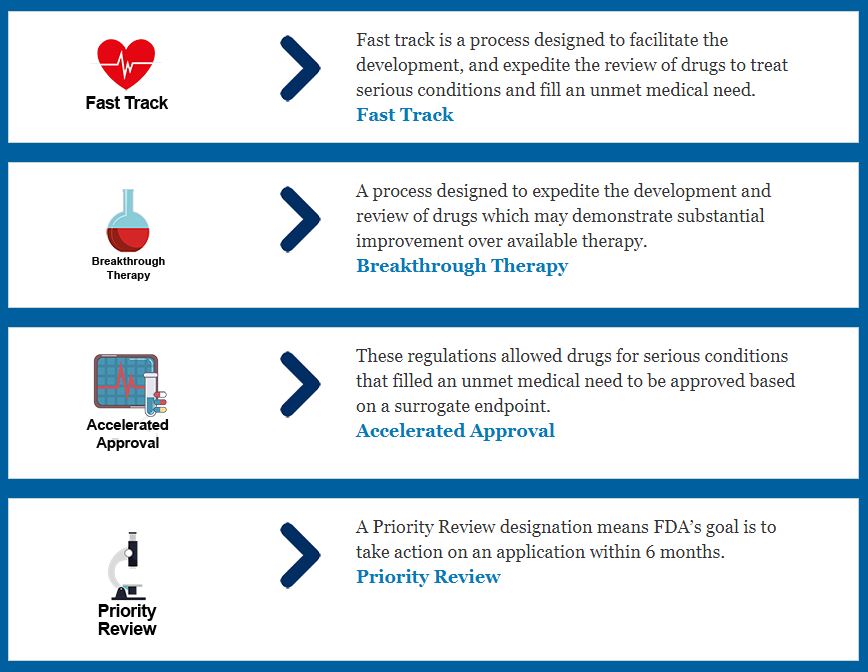
Drug Development Process
Brexpiprazole Warnings: Side Effects of Rexulti

FDA rushes approval of dementia drug that quadruples risk of death

Regulatory - Page 7 of 12 - Drug Discovery World (DDW)

FDA Approves Rexulti for Agitation Associated With Dementia Due to Alzheimer's Disease

Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation
Recomendado para você
-
REXULTI® (brexpiprazole), MDD04 setembro 2024
-
Brexpiprazole (Rexulti): Uses, Side Effects, Warnings & More - GoodRx04 setembro 2024
-
Rexulti Full Prescribing Information, Dosage & Side Effects04 setembro 2024
-
 REXULTI® (brexpiprazole) agitation that may happen with dementia due to Alzheimer's disease04 setembro 2024
REXULTI® (brexpiprazole) agitation that may happen with dementia due to Alzheimer's disease04 setembro 2024 -
 Rexulti Brexpiprazole 1mg Tablet, 30 Tablets, Treatment: Schizophrenia, Depression04 setembro 2024
Rexulti Brexpiprazole 1mg Tablet, 30 Tablets, Treatment: Schizophrenia, Depression04 setembro 2024 -
 Rexulti 1mg Lundbeck 30 Comprimidos - Drogaria Sao Paulo04 setembro 2024
Rexulti 1mg Lundbeck 30 Comprimidos - Drogaria Sao Paulo04 setembro 2024 -
 Rexulti Patient Site - Once Daily Pharma04 setembro 2024
Rexulti Patient Site - Once Daily Pharma04 setembro 2024 -
 Precio Rexulti 1 mg 10 con tabletas04 setembro 2024
Precio Rexulti 1 mg 10 con tabletas04 setembro 2024 -
 REXULTI® (Brexpiprazole) FDA Approved for Treating Agitation in Alzheimer's Disease Patients04 setembro 2024
REXULTI® (Brexpiprazole) FDA Approved for Treating Agitation in Alzheimer's Disease Patients04 setembro 2024 -
 REXULTI- brexpiprazole tablet04 setembro 2024
REXULTI- brexpiprazole tablet04 setembro 2024
você pode gostar
-
 New 'game ranch' development causing concerns among some in Madison County - East Idaho News04 setembro 2024
New 'game ranch' development causing concerns among some in Madison County - East Idaho News04 setembro 2024 -
 Receita de Fígado Bovino Frito, DeliRec04 setembro 2024
Receita de Fígado Bovino Frito, DeliRec04 setembro 2024 -
 CODE) RENGOKU VS Every DEMON In Project Slayers Update 1.504 setembro 2024
CODE) RENGOKU VS Every DEMON In Project Slayers Update 1.504 setembro 2024 -
 Speculation rolls for GTA 6's price hike, maps, and advanced AI04 setembro 2024
Speculation rolls for GTA 6's price hike, maps, and advanced AI04 setembro 2024 -
 Tanjiro meme, tanjiro , meme , anime , manga , demon , slayer - Free PNG - PicMix04 setembro 2024
Tanjiro meme, tanjiro , meme , anime , manga , demon , slayer - Free PNG - PicMix04 setembro 2024 -
 TERMOS TÉCNICOS DE TEATRO – Escola de Teatro Juliana Leite04 setembro 2024
TERMOS TÉCNICOS DE TEATRO – Escola de Teatro Juliana Leite04 setembro 2024 -
 How to watch the 2022 FIFA World Cup in Houston04 setembro 2024
How to watch the 2022 FIFA World Cup in Houston04 setembro 2024 -
 Naruto vai morrer? Criador da série discute morte de personagem em Boruto - 08/05/2017 - UOL Start04 setembro 2024
Naruto vai morrer? Criador da série discute morte de personagem em Boruto - 08/05/2017 - UOL Start04 setembro 2024 -
Jewels & Genies: Aladdin Quest – Apps no Google Play04 setembro 2024
-
 Lista nowości w Player.pl – dodano 50 tytułów! 02.06.202104 setembro 2024
Lista nowości w Player.pl – dodano 50 tytułów! 02.06.202104 setembro 2024