Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 07 julho 2024

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

IHEP (International Hepatology Education Program)

Journal of Medicinal Chemistry
EuroBiotech Report: Woodford's loss, BioInvent's major failure

Hepatitis B Foundation

Heplisav-B: A New Hepatitis B Vaccine That Can Be Used For Pre

Can Medivation's CEO get a bidding war started?

Heart trouble report & clinical hold spell end of Antios, Assembly

IHEP (International Hepatology Education Program)

Robust hepatitis B vaccine-reactive T cell responses in failed
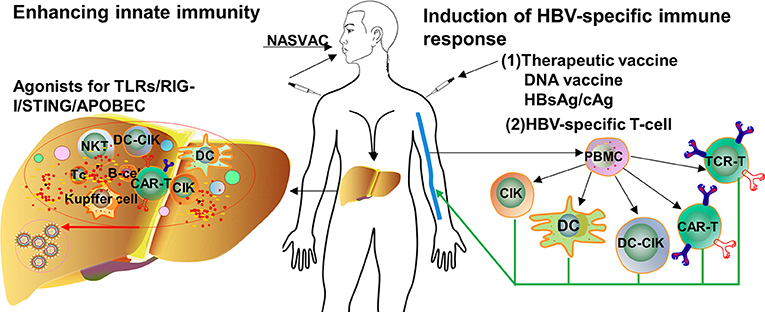
Frontiers Advances in Targeting the Innate and Adaptive Immune

Annalee Armstrong - Journalist Profile - Intelligent Relations
Recomendado para você
-
 Paint Spray Booths: Construction, Types, Applications, and Benefits07 julho 2024
Paint Spray Booths: Construction, Types, Applications, and Benefits07 julho 2024 -
 whos your favorite monster in doors|TikTok Search07 julho 2024
whos your favorite monster in doors|TikTok Search07 julho 2024 -
 The Office Is Dead. Get ready for the commercial real…, by Courtney Rubin07 julho 2024
The Office Is Dead. Get ready for the commercial real…, by Courtney Rubin07 julho 2024 -
 LED Tail Light Set, Early Bronco07 julho 2024
LED Tail Light Set, Early Bronco07 julho 2024 -
 The Anatomy of a Sh*tco, Energy Edition - by Broncho07 julho 2024
The Anatomy of a Sh*tco, Energy Edition - by Broncho07 julho 2024 -
 American Guns Drive the Migrant Crisis That Trump Wants to Fix With a Wall07 julho 2024
American Guns Drive the Migrant Crisis That Trump Wants to Fix With a Wall07 julho 2024 -
 53 Best Royal Moments of the 2010s - Royal Moments of the Decade07 julho 2024
53 Best Royal Moments of the 2010s - Royal Moments of the Decade07 julho 2024 -
 GMC Jam Voting - The GMC Jam 46, Voting Topic07 julho 2024
GMC Jam Voting - The GMC Jam 46, Voting Topic07 julho 2024 -
 How to Become a Healthy Team (All You Need to Know in 30 Minutes07 julho 2024
How to Become a Healthy Team (All You Need to Know in 30 Minutes07 julho 2024 -
 NTSB wants halt on doors-off sightseeing helicopter rides07 julho 2024
NTSB wants halt on doors-off sightseeing helicopter rides07 julho 2024
você pode gostar
-
 Naruto Shippuden: Ultimate Ninja Storm 4 Guide - IGN07 julho 2024
Naruto Shippuden: Ultimate Ninja Storm 4 Guide - IGN07 julho 2024 -
 Linguagens, Códigos e suas Tecnologias – Educação Física Ensino Fundamental, 8º Ano U Abordagem histórica dos jogos populares, de salão e esportivos Participação: - ppt carregar07 julho 2024
Linguagens, Códigos e suas Tecnologias – Educação Física Ensino Fundamental, 8º Ano U Abordagem histórica dos jogos populares, de salão e esportivos Participação: - ppt carregar07 julho 2024 -
 04 dicas para praticar o inglês em casa – PES07 julho 2024
04 dicas para praticar o inglês em casa – PES07 julho 2024 -
 𝐲𝐮𝐤𝐢-𝐨𝐧𝐧𝐚 𝐛𝐲 @𝐫𝐢𝐧𝐜𝐚𝐩𝐬 𝐨𝐧 𝐩𝐢𝐧𝐭𝐞𝐫𝐞𝐬𝐭 em07 julho 2024
𝐲𝐮𝐤𝐢-𝐨𝐧𝐧𝐚 𝐛𝐲 @𝐫𝐢𝐧𝐜𝐚𝐩𝐬 𝐨𝐧 𝐩𝐢𝐧𝐭𝐞𝐫𝐞𝐬𝐭 em07 julho 2024 -
 This 3-Game PS4 Bundle Is Cheaper Than Buying the Console Alone07 julho 2024
This 3-Game PS4 Bundle Is Cheaper Than Buying the Console Alone07 julho 2024 -
 Smash or Pass: All 898 Pokémon07 julho 2024
Smash or Pass: All 898 Pokémon07 julho 2024 -
 File:Chevrolet Corsa 1.6 Sedan 2010 (16357782189).jpg - Wikipedia07 julho 2024
File:Chevrolet Corsa 1.6 Sedan 2010 (16357782189).jpg - Wikipedia07 julho 2024 -
 Premium Vector Fried egg sunny side up eggs vector flat design realistic illustration art07 julho 2024
Premium Vector Fried egg sunny side up eggs vector flat design realistic illustration art07 julho 2024 -
 BUBBLES - Play Online for Free!07 julho 2024
BUBBLES - Play Online for Free!07 julho 2024 -
 The Sims Freeplay Dinheiro Infinito Vip 2023 Apk Mod v5.81.0 - W07 julho 2024
The Sims Freeplay Dinheiro Infinito Vip 2023 Apk Mod v5.81.0 - W07 julho 2024