hydrogen orbital wavefunction
Por um escritor misterioso
Last updated 05 agosto 2024
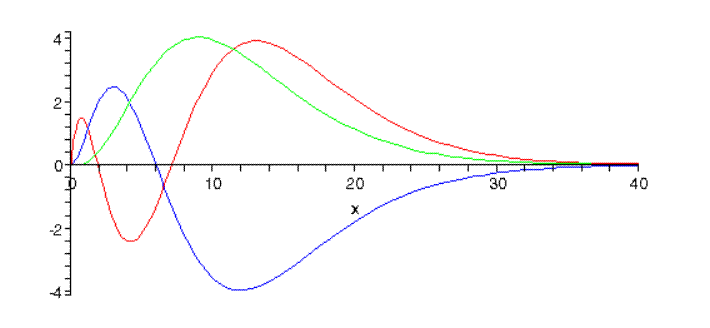
Hydrogen Atom

The wave function for 2s orbital is given as: `Psi = ((1)/(sqrt2)) ((1)/(alpha_(0)))^(3//2)(2- (r)

Hydrogen-like atom - Wikipedia

11.10: The Schrödinger Wave Equation for the Hydrogen Atom - Chemistry LibreTexts

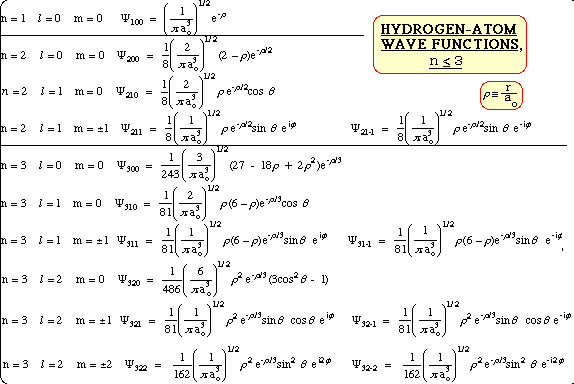
Schrodinger's Wave Equation for Hydrogen Atom
For the Hydrogen atomic 2s wave function given by psi = 1/(2sqrt(2pi)) sqrt(1/a_0) (2 - r/a_0)e^(-r//2a_0), at what radial distance away from the nucleus can no electrons be found?
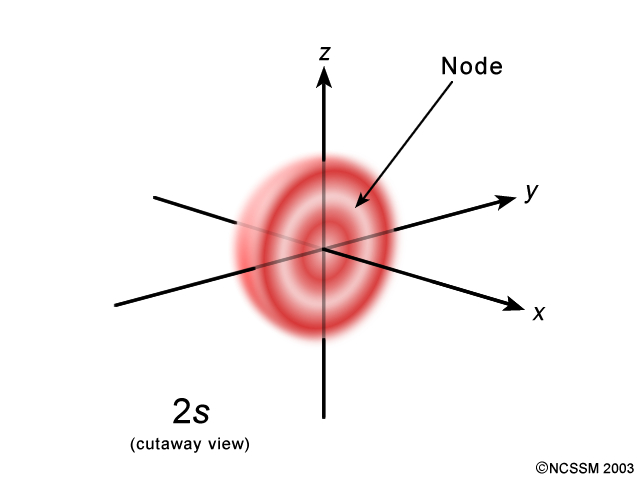
How were the shapes of s, p, d, and f orbitals determined? How did they get their names of s, p, d, and f?
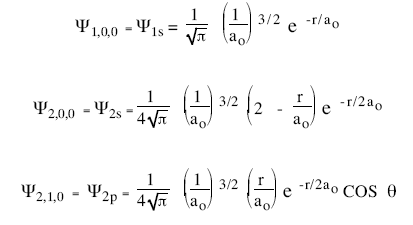
The Hydrogen Atom

Hydrogen Atom Wave Function Electron Orbital (3,2,0) Poster for Sale by cbeuw
Recomendado para você
-
Gacha Life Club Wallpaper Cute - Apps on Google Play05 agosto 2024
-
 Pin on Salvamentos rápidos05 agosto 2024
Pin on Salvamentos rápidos05 agosto 2024 -
 tela verde animação boca|Pesquisa do TikTok05 agosto 2024
tela verde animação boca|Pesquisa do TikTok05 agosto 2024 -
Pavilion Nordico05 agosto 2024
-
Fotos de gacha life :v05 agosto 2024
-
 gacha #gachaclub #gachastudio #gachaclubedit #gachaedit #gachaedits # gachalife05 agosto 2024
gacha #gachaclub #gachastudio #gachaclubedit #gachaedit #gachaedits # gachalife05 agosto 2024 -
 ashkittycat - Student, General Artist05 agosto 2024
ashkittycat - Student, General Artist05 agosto 2024 -
 Marfa Drive-In - Büro Ole Scheeren05 agosto 2024
Marfa Drive-In - Büro Ole Scheeren05 agosto 2024 -
![✨How To Draw GACHA Life Mouths✨[ How I draw mouths GACHA life ] Gacha Life Tutorial! [ GIVE CREDIT ]](https://i.ytimg.com/vi/cIrPA9z7d0U/maxresdefault.jpg) ✨How To Draw GACHA Life Mouths✨[ How I draw mouths GACHA life ] Gacha Life Tutorial! [ GIVE CREDIT ]05 agosto 2024
✨How To Draw GACHA Life Mouths✨[ How I draw mouths GACHA life ] Gacha Life Tutorial! [ GIVE CREDIT ]05 agosto 2024 -
 sajapxlr Profiles Desenho de lábios, Como desenhar um nariz05 agosto 2024
sajapxlr Profiles Desenho de lábios, Como desenhar um nariz05 agosto 2024
você pode gostar
-
 Guess The Football Team - Football Quiz 2021 APK for Android - Download05 agosto 2024
Guess The Football Team - Football Quiz 2021 APK for Android - Download05 agosto 2024 -
![100+] 1080p Gta 5 Wallpapers](https://wallpapers.com/images/featured/1080p-gta-5-f500vqmcsqkt7hzh.jpg) 100+] 1080p Gta 5 Wallpapers05 agosto 2024
100+] 1080p Gta 5 Wallpapers05 agosto 2024 -
 Ingresso de Vantagens05 agosto 2024
Ingresso de Vantagens05 agosto 2024 -
/i.s3.glbimg.com/v1/AUTH_59edd422c0c84a879bd37670ae4f538a/internal_photos/bs/2023/P/t/q4jPTeSBW7J7AyAl6ZYA/estudantes-puderam-experimentar-as-armacoes-dos-oculos-que-serao-entregues-gratuitamente-foto-bruno-campos-prefeitura-de-macae.jpg) Alunos de escolas públicas de Macaé vão ganhar óculos por meio do05 agosto 2024
Alunos de escolas públicas de Macaé vão ganhar óculos por meio do05 agosto 2024 -
 Dark Crypto Lady 🦊 on X: PSA: Be careful about clicking links in05 agosto 2024
Dark Crypto Lady 🦊 on X: PSA: Be careful about clicking links in05 agosto 2024 -
Steam Workshop::Spetsnaz [Counter-Strike: Condition Zero Deleted05 agosto 2024
-
 Decoração de Cartaz Anime Fire Force, Cartazes Home, Parede do Quarto, Papel Kraft Pictur, Impressões Retro, Arte, Bar, Café, Adesivos05 agosto 2024
Decoração de Cartaz Anime Fire Force, Cartazes Home, Parede do Quarto, Papel Kraft Pictur, Impressões Retro, Arte, Bar, Café, Adesivos05 agosto 2024 -
King of the Hill is the best anime05 agosto 2024
-
 Iza aposta em vestido com flores de crochê no 'The Voice Kids': Barbie05 agosto 2024
Iza aposta em vestido com flores de crochê no 'The Voice Kids': Barbie05 agosto 2024 -
 Historia de la Liga no “Uruguaya” de Fútbol » Diario Uruguay05 agosto 2024
Historia de la Liga no “Uruguaya” de Fútbol » Diario Uruguay05 agosto 2024



